|
1. 
Glucosinolates
are a class of about 100 naturally occurring thioglycosides (play
numerous important roles in living organisms) that are
characteristic of the Cruciferae and related families in the order Capparales.
(is a botanical name of an order of flowering plants)
At the present time the diets of people in many parts of
the world include considerable amounts of Cruciferous crops and
plants. These range from the consumption of processed radish and wasabi in the Far East to that of cabbage and traditional root
vegetables in Europe and North America. Other crops, such as
rapeseed, kale, swede and turnip may also contribute indirectly to
the human food chain since they are extensively used as animal feed
stuff. The presence of some glucosinolates in agricultural crop
plants, such as oilseed rape (Brassica napus) and Brassica
vegetables, is undesirable because of the toxicological effects of
their breakdown products. Such breakdown products include nitriles,
isothiocyanates, thiocyanates, epithionitriles and vinyl
oxazolidinethiones. Some glucosinolates, especially those in
broccoli, have anticarcinogenic properties and are being studied for
their potential therapeutic use. Glucosinolate breakdown products
are responsible for the biting taste of important condiments such as
horseradish and mustard, and they contribute to the characteristic
flavours of many vegetables, such as cabbage, broccoli and
cauliflower.
The biological role of glucosinolates and their degradation
products is not completely understood. The enzymes catalysing the
hydrolysis of glucosinolates are known as myrosinases. The
complexity of the myrosinase-glucosinolate system indicates an
important role in the life cycle of plants. The function of this
system may be diverse. The glucosinolates may be a sink for
nutrients like nitrogen and sulphur, while the products of
hydrolysis may have important roles in the plant defense system
against insect, fungi and microorganism infections.
Plant breeding strategies have concentrated on reducing the glucosinolate content of rape seeds. Seeds with very low
glucosinolate content have been processed, but there has been a
significant cost in terms of crop protection and nutrition.
Modulation of the biosynthesis of specific glucosinolates is a major
goal in Brassica breeding.
2. 
The
glucosinolates are a class of secondary metabolites found in
fifteen botanical families of dicotyledonous plants. These families
are the Akaniaceae, Bataceae, Brassicaceae, Bretschneideraceae,
Capparaceae, Caricaceae, Euphorbiaceae, Gyrostemonaceae,
Limnanthaceae, Moringaceae, Pentadiplantdraceae, Resedaceae,
Salvodoraceae, Tropaeolaceae and Tovariaceae. At the present time
over 100 glucosinolates have been reported. Glucosinolates are found
in all parts of the plant and up to fifteen different glucosinolates
have been found in the same plant. Generally, levels in the seed are
high (up to ten per cent of the dry weight), whereas the levels in
the leaf, stem and root are approximately ten times lower.
Concentrations differ according to tissue type, physiological age,
plant health and nutrition.
Studies have shown that myrosinases are localised in vacuoles of
specialised plant cells, called myrosin cells. Thus the two
components of the system are separated until autolysis or tissue
damage brings them into contact. The precise localization of
glucosinolates is not known, but they have been reported to be
stored in vacuoles.
3. 
The skeleton of
glucosinolates consists of a thioglycosides link to the carbon of a
sulphonated oxime. The R group (side chain) and the sulphate group
have anti stereochemical configuration. The R group is derived from
amino acids and is highly variable. It can be aliphatic (e.g. alkyl,
alkenyl, hydroxyalkenyl, w-methylthioalkyl), aromatic (e.g. benzyl,
substituted benzyl) or heterocyclic (e.g. indolyl). The sulphate
group imparts strongly acidic properties and thus the glucosinolates
occur in nature as anions counterbalanced by a cation. The cation
is usually potassium, being one of the most abundant cations in
plant tissues. The sulphate group and the thioglucose moiety impart
nonvolatile and hydrophilic properties to all glucosinolates, the R
group is variable in properties from lipophilic to marked
hydrophilic. The natural forms of glucosinolates exhibit laevo
rotation in solution. Glucosinolates have a large number of
homologues and ß-hydroxylated analogues. As an example w-methylthioalkyl
side chains range from MeS(CH2)3 to MeS(CH2)8.
The general structure of glucosinolates is shown in figure 1.

Figure 1. 
When
glucosinolates were first discovered they were named after the
plants in which they were found. With the discovery of more
glucosinolates a semi-systematic system for their naming arose,
based on the structure of the side chain. Table 1 shows trivial
names for some glucosinolates and indicates their side chain. The
name of the side chain followed by the word "glucosinolate" gives
the semi-systematic name. The suffix "ate" indicates the anionic
nature of glucosinolates.
Table 1. Otherl Names and
Their Side
Chain for Some Glucosinolates
|
Trivial name |
Side chain |
|
Gluconasturtiin |
2-Phenethyl |
|
Glucotropaeolin |
Benzyl |
|
Progoitrin\epiprogoitrin |
2-hydroxy-3-butenyl |
|
Sinigrin |
2-propenyl |
|
(Gluco)sinalbin |
p-Hydroxybenzyl |
4. 
When crushed plant tissue or seeds containing glucosinolates are
added to water, myrosinases catalyse the hydrolytic cleavage of the
thioglucosidic bond, giving D-glucose and a
thiohydroximate-O-sulphonate (aglycone). The latter compound
rearranges nonenzymatically with release of sulphate to give one of
several possible products. The predominant product is dependent on
the structure of the glucosinolate side chain and the presence of
protein co-factors that modify the action of the enzyme. The most
frequent fate of the unstable aglycone is to undergo rearrangement
spontaneously via a proton independent Lossen rearrangement with a
concerted loss of sulphate to yield an isothiocyanate, or a
competing proton dependent desulphuration yielding a nitrile and
elemental sulphur. Some glucosinolates also give rise to the
formation of thiocyanates.

A mixture of products is normally formed. At low pH the
formation of the nitrile is favoured, whereas neutral or high pH
favours the formation of the isothiocyanate by means of the Lossen
rearrangement. The Lossen rearrangement is characterised by the
migration of the nitrogen atom and subsequent loss of the sulphate
group. Glucosinolates with a beta-hydroxylated side chain yield
isothiocyanates which undergo spontaneous cyclization to the
corresponding oxazolidone-2-thione. An example of this is the
formation of goitrin from the glucosinolate progoitrin.
The addition of ferrous ions to reaction mixtures promotes the
formation of the nitrile hydrolysis product. At low pH, a proton may
block the Lossen rearrangement of the aglycone, thus promoting
formation of the nitrile. It is thought that the ferrous ion may
serve a similar function. Fe2+ may act by complexing
ascorbic acid, a co-factor of some myrosinase isoenzymes, thus
rendering it unavailable to the isoenzyme.
Epithiospecifier protein, ESP, is a small protein of molecular
weight 30 to 40 kDa, which co-occurs with myrosinase. ESP does not
have thioglucosidase activity, but interacts with myrosinase to
promote the transfer of sulphur from the S-glucose moiety of
terminally unsaturated glucosinolates to the alkenyl moiety,
resulting in the formation of epithionitriles. The presence of
ferrous ions are essential for ESP function. Enzyme characteristics
(substrate affinity, temperature and pH optima) may alter relative
proportions of products by causing some glucosinolates to be
hydrolyzed at different rates.
5. 
Studies have shown that
glucosinolates are derived from amino acids. The biosynthetic
studies have involved feeding experiments with labeled compounds,
isolation of intermediates and isolation of some of the enzymes
involved in the pathway. Aliphatic, indole and aromatic side chains
are derived from methionine, tryptophan and phenylalanine
respectively, from both protein and non-protein sources. The initial
steps in the formation of most glucosinolates are N-hydroxylation
followed by oxidative decarboxylation to yield an aldoxime. These
steps are common to the biosynthesis of other groups of natural
products. The biosynthetic pathways then diverge at the aldoxime to
produce different compounds. The majority of glucosinolates possess
aglycone structures which are not related to protein amino acids. It
is generally accepted, however, that these glucosinolates are also
derived from protein amino acids. These protein amino acids undergo
a chain elongation process in which their 2-oxo-acids condense with
acetate. The entire homologous series of glucosinolates with side
chains ranging from R= MeS(CH2)3 to R=MeS(CH2)8
is considered to be derived from repeated chain extensions starting
with methionine. Each time the sequence is traversed a new higher
amino acid homologue is formed. A glyoxylate aminotransferase is
believed to be the first enzyme of the chain elongation process.
This enzyme catalyses the formation of the 2-oxo-acid from its
corresponding amino acid.
All the intermediates between the amino acid and the glucosinolate are nitrogenous and the amino acid carbon-nitrogen
bond is preserved. The amino acid, whether it has undergone chain
elongation or not, is specifically hydroxylated to the N-hydroxyamino
acid in the presence of oxygen and NADPH. The N-hydroxyamino acid is
decarboxylated to give the aldoxime, followed by a reduction step to
a nitro compound which tautomerises to the aci form. The
thiohydroximate is then formed by introduction of sulphur, feeding
experiments have shown that cysteine is involved as the sulphur
donor. The thiohydroximate is transglycosylated to the
desulphoglucosinolate. An enzyme catalysing the transfer of glucose
from UDP- glucose to the thiohydroximate has been isolated. The
glucosinolate is obtained by sulphonation and it is known
3`-phosphoadenosine-5`-phosphosulphate (PAPS) is involved as the
sulphate donor. A summary of the biosynthesis of glucosinolates is
shown in figure 3.
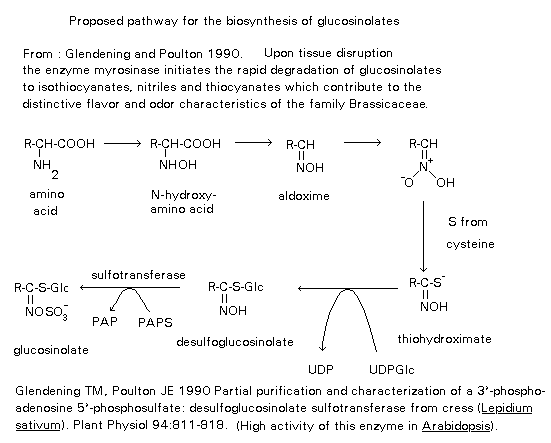
Figure 3. 
Important modifications of
the side chain, such as hydroxylation and oxidative generation of
the alkene from the methylthio group, occur after transglycosylation.
It is clear that a number of important details, including possible
enzymes involved in the early stages of the glucosinolate
biosynthesis, needs to be further researched.
|